| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |
|
| ||||||||
.gif)
* Department of Animal and Dairy Science, University
of Georgia, Athens 30602 Animal Improvement Programs
Laboratory, Agricultural Research Service, USDA, Beltsville, MD
20705
Animal Improvement Programs
Laboratory, Agricultural Research Service, USDA, Beltsville, MD
20705.gif) Holstein Association USA Inc., Brattleboro, VT 05301
Holstein Association USA Inc., Brattleboro, VT 05301
1 Corresponding author: jbohmano{at}uoguelph.ca
Heat stress was evaluated as a factor in differences between
regional evaluations for milk yield in the United States. The
national data set (NA) consisted of 56 million first-parity,
test-day milk yields on 6 million Holsteins. The Northeastern
subset (NE) included 12.5 million records on 1.3 million first-calved
heifers from 8 states, and the Southeastern subset (SE) included
3.5 million records on 0.4 million heifers from 11 states. Climatic
data were available from 202 public weather stations. Each herd
was assigned to the nearest weather station. Average daily
temperature-humidity index (meanTHI) 3 d before test date was used as
an indicator of heat stress. Two test-day repeatability models were
implemented. Effects included in both models were herd-test date, age
at calving class, frequency of milking, days in milk x season class, additive genetic (regular
breeding value) and permanent environmental effects. Additionally,
the second model included random regressions on degrees of heat
stress (t = max[0, meanTHI – 72]) for additive genetic (breeding
value for heat tolerance) and permanent environmental effects. Both
models were fitted with the national and regional data sets.
Correlations involved estimated breeding values (EBV) from SE and NE
for sires with 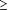 100 and
100 and 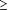 300 daughters in each
region. When heat stress was ignored (first model) the correlations
of regular EBV between SE and NE for sires with
300 daughters in each
region. When heat stress was ignored (first model) the correlations
of regular EBV between SE and NE for sires with 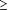 100 (
100 (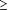 300) daughters were 0.85 (0.87). When heat
stress was considered (second model), the correlation increased by up
to 0.01. The correlations of heat stress EBV between NE and SE for
sires with
300) daughters were 0.85 (0.87). When heat
stress was considered (second model), the correlation increased by up
to 0.01. The correlations of heat stress EBV between NE and SE for
sires with 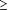 100 (
100 (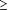 300,
300, 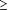 700) daughters were 0.58 (0.72, 0.81).
Evaluations for heat tolerance were similar in cooler and hotter
regions for high-reliability sires. Heat stress as modeled
explains only a small amount of regional differences, partly
because test-day records depict only snapshots of heat stress.
700) daughters were 0.58 (0.72, 0.81).
Evaluations for heat tolerance were similar in cooler and hotter
regions for high-reliability sires. Heat stress as modeled
explains only a small amount of regional differences, partly
because test-day records depict only snapshots of heat stress.
Key Words: genotype x environment interaction • reaction norm • heat stress
| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |